Impulsionadas pela RDC 54/2013 da ANVISA, as normas de rastreabilidade, que englobam os diversos elos dessa cadeia (fabricante, fornecedor, distribuidor, transportador, comprador e consumidor) passaram a ser discutidas, mas não definidas.
Diante da indefinição, os hospitais tomaram iniciativas para minimizar os erros de administração medicamentosa, já que muitas etapas fazem parte deste processo: identificação correta, prescrição, distribuição, dispensação, monitoramento e uso, montando verdadeiras linhas de produção de doses unitarizadas, o que gerou um aumento de custos com equipamentos e pessoas para executarem esse trabalho.
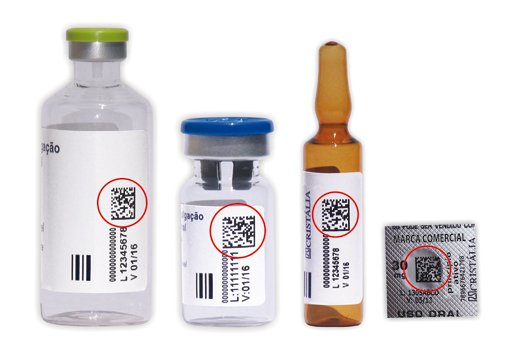
A tecnologia Datamatrix foi definida pela RDC 54, publicada em dezembro de 2013, como a tecnologia de captura, armazenamento e transmissão eletrônica de dados necessários ao rastreamento de medicamentos no Brasil.
A inclusão do código de barras nas embalagens secundárias ficou a cargo das empresas detentoras de registro de medicamentos, que tinham um prazo de três anos, a partir da publicação da RDC, para atender à resolução.
O DNA inovador e o foco no segmento Hospitalar, somados à velocidade e à capacidade de execução, permitiram que, em curto tempo, o Cristália atendesse às necessidades apontadas, superando as expectativas dos hospitais brasileiros, colocando o código Datamatrix em suas embalagens primárias.
As inovações desenvolvidas pelo Cristália têm diferenciais tecnológicos que promovem eficácia e segurança, resultando em ganho terapêutico para a toda a cadeia da saúde no Brasil.